Abstract
Background: Half of adults with newly diagnosed multiple myeloma (NDMM) in the US are ≥70y. This age group is often considered ineligible for intensive therapy and not routinely included in prospective clinical trials. The phase II MASTER trial (NCT03224507) for NDMM did not have an upper age limit as eligibility and showed high rates of minimal residual disease- (MRD) negativity among NDMM patients receiving quadruplet induction, autologous stem cell transplantation (ASCT) and response-adapted consolidation therapy (Costa LJ et al JCO 2021). Here, we conducted a post-hoc analysis comparing the outcomes of patients age ≥70y. vs <70y.
Methods: The MASTER trial enrolled patients with NDMM, with ECOG ≤2, measurable disease and creatinine clearance ≥40 ml/min as key eligibility criteria. Patients received Daratumumab, Carfilzomib, Lenalidomide and Dexamethasone (Dara-KRD based induction, ASCT and response adapted Dara-KRD consolidation. The primary end point was achievement of MRD-negativity (<10-5) at any point during follow-up by NGS (ClonoSEQ®). Secondary endpoints included achievement of MRD <10-6 at any point, rate of MRD negativity post-induction, rate of complete response, progression-free survival (PFS) and overall survival (OS). Those who achieved two consecutive MRD-negative assessments underwent treatment free observation and MRD surveillance (MRD-SURE). For the current analysis, we compared the achievement of primary and secondary endpoints using intent-to-treat principle between the study cohorts.
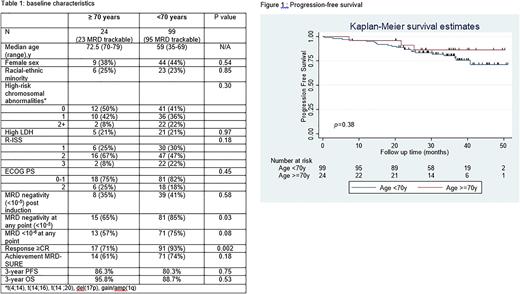
Results: Of the 123 enrolled patients, 24 (19.5%) were ≥70y at the time of diagnosis (5 were ≥75y). Baseline characteristics were similar between the two age groups (Table 1). All 24 patients received planned ASCT. Compared to younger adults, older adults had similar rates of MRD negativity post induction (36% vs 41%; p= 0.66), but lower rates of overall MRD negativity (<10-5) (65% vs 84%; p=0.03) and lower rates of complete response (71% vs 93%; p= 0.002) (Table 1). Older and younger patients had similar probability of achieving MRD-SURE (61% vs. 74%, P=0.18). Median time on protocol treatment was 11.7 (IQR 7.7-15.2) months (m) for older patients and 11.6 (IQR 8.0-14.7) m for younger patients (p=0.85). Grade ³3 treatment emergent adverse events occurred in 79% of older and 69% of younger patients (p=0.31). No patients in the older group permanently discontinued protocol therapy due to toxicity. At a median follow up of 35.9 (range 1-51) m, older vs younger adults had similar PFS (3-y PFS 86.3% vs 80.3%; p= 0.74)(Fig) and OS (3y OS 95.8% vs 88.7%; p= 0.53). Three deaths occurred during the treatment period, of which one patient was ≥70y (unwitnessed sudden death 2 m after ASCT and before consolidation).
Conclusion: Our study demonstrates that older adults can be candidates for quadruplet induction, ASCT, and MRD-adapted consolidation therapy and can derive similar benefits as younger NDMM pts. While toxicity may be higher among older adults, it does not appear to compromise treatment continuation, PFS or OS. Chronologic age, by itself, should not be an eligibility criterion from trials utilizing higher intensity therapies. Future studies should focus on how to identify older adults who can benefit from intensive frontline MRD adapted therapies.
Disclosures
Giri:CareVive: Honoraria, Research Funding; OncLive: Honoraria; Pack Health: Research Funding. Chhabra:Janssen: Research Funding; GlaxoSmithKline: Honoraria; Sanofi: Research Funding; Amgen: Research Funding. Dholaria:BMS: Research Funding; BEAM Therapeutics: Consultancy; Pfizer: Research Funding; Janssen: Research Funding; Vanderbilt University Medical Center: Current Employment; Arivan: Consultancy; Takeda: Research Funding; Poseida: Research Funding; Angiocrine: Research Funding; Jazz Pharmaceuticals: Consultancy; MEI Pharma: Research Funding; Molecular Templates: Research Funding; Wugen: Research Funding; MJH Biosciences: Honoraria; Gamida Cell: Consultancy; Orca Bio: Research Funding. Schmidt:Sanofi: Consultancy; Janssen: Consultancy. Silbermann:Sanofi-Aventis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees. Dhakal:Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Natera: Consultancy; Karyopharm Therapeutics: Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Research Funding, Speakers Bureau; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; BMS: Honoraria, Research Funding; Arcellx: Research Funding; Carsgen: Research Funding; Cartesian: Research Funding; Fate: Research Funding; Takeda: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees. D'Souza:Pfizer, Janssen Oncology, Bristol-Myers Squibb/Celgene, Prothena: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda, Sanofi, TeneoBio, Prothena, Caelum Biosciences, Janssen Oncology, Regeneron, Abbvie: Research Funding. Cornell:Abbvie: Current Employment, Current holder of stock options in a privately-held company. Hari:Spectrum Pharmaceuticals: Research Funding; Millennium: Research Funding; Pharmacyclics: Consultancy; Takeda: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Novartis: Honoraria; AbbVie: Honoraria; GlaxoSmithKline: Honoraria; Kite: Consultancy, Honoraria; Incyte: Honoraria; Iovance: Current Employment. Costa:Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Adaptive Biotechnologies: Consultancy, Honoraria; AbbVie: Research Funding; Genentech: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal